High temperature reactions because a styraphoam container is going to melt in high temperature so were not going to use that in high temperatures. A bomb calorimeter operates in the same practice as a coffee cup calorimeter with one big variation. Coffee cup calorimeter vs bomb calorimeter.
Coffee Cup Calorimeter Vs Bomb Calorimeter, In a coffee cup calorimeter the reaction takes place in the water. Since the coffee cup calorimeter has constant pressure it gives us enthalpy values. A bomb calorimeter is used to measure heat flow for solids with low to high temperature reactions. The coffee cup calorimeter cant be used for high temperature reactions either since these would melt the cup.
 Calorimeter Physique Chimie Chimie From pinterest.com
Calorimeter Physique Chimie Chimie From pinterest.com
View Homework Help - bomb calorimetrydocx from CHEM 111 at University of South Carolina. Primarily a calorimeter is a tool utilized to estimate both specific heat capacity or the quantity of energy generated or consumed in a chemical reaction. But bomb calorimeter is also used in high temperature reactions for processes. We have the thermometer and the reaction mixture which are self explanatory along with the stirrer which stirs the reaction mixture.
CalorimetryChemistry AbdullahSanaullahTutorials Calorimetry CalorimeterCoffee CupCalorimeterBombCalorimeterClass11 NEET JEE MDCAT ECAT BestVi.
Read another article:
CalorimetryChemistry AbdullahSanaullahTutorials Calorimetry CalorimeterCoffee CupCalorimeterBombCalorimeterClass11 NEET JEE MDCAT ECAT BestVi. We have the thermometer and the reaction mixture which are self explanatory along with the stirrer which stirs the reaction mixture. What is the difference between a coffee cup calorimeter and a bomb calorimeter in terms of the parameters. But bomb calorimeter is also used in high temperature reactions for processes. What is the difference between bomb calorimeter and coffee cup calorimetry.
 Source: pinterest.com
Source: pinterest.com
In a coffee cup calorimeter the reaction takes place in the water. The bomb calorimeter is just more advanced in that it is capable of withstanding more pressure from a reaction that may occur in the constant volume system. What is the difference between bomb calorimeter and coffee cup calorimetry. Postby Jessica Benitez 1K Mon Jan 15 2018 712 am The bomb calorimeter has constant volume while the coffee cup calorimeter has constant pressure. Calorimeters And Calorimetry Physics Classroom Physics Concepts Physics.
 Source: in.pinterest.com
Source: in.pinterest.com
The coffee cup calorimeter cant be used for high temperature reactions either since these would melt the cup. The coffee cup calorimeter is a a simplified DIY calorimeter but both serve the same purpose. View Homework Help - bomb calorimetrydocx from CHEM 111 at University of South Carolina. Top 3 posts Page 1 of 1 Return to Heat Capacities Calorimeters Calorimetry Calculations Jump to. Where Is A Bomb Calorimetr Used Dds Calorimeters These Are Things Such As Oil Analysis Coal Analysis Nutritional Research Analysis Animal Bombs Dds Oils.
 Source: pinterest.com
Source: pinterest.com
What explains the key difference between a bomb calorimeter and a. The coffee cup calorimeter cant be used for high temperature reactions either since these would melt the cup. Also why couldnt you just perform the former in a bomb calorimeter. A bomb calorimeter operates in the same practice as a coffee cup calorimeter with one big variation. Speed Regulated Polishing Machine India Speed Regulated Polishing Machine Manufacturer Speed Regulated Polishing Machine Suppliers And Speed Regulated Polishi Regulators Lab Equipment Machine.
 Source: pinterest.com
Source: pinterest.com
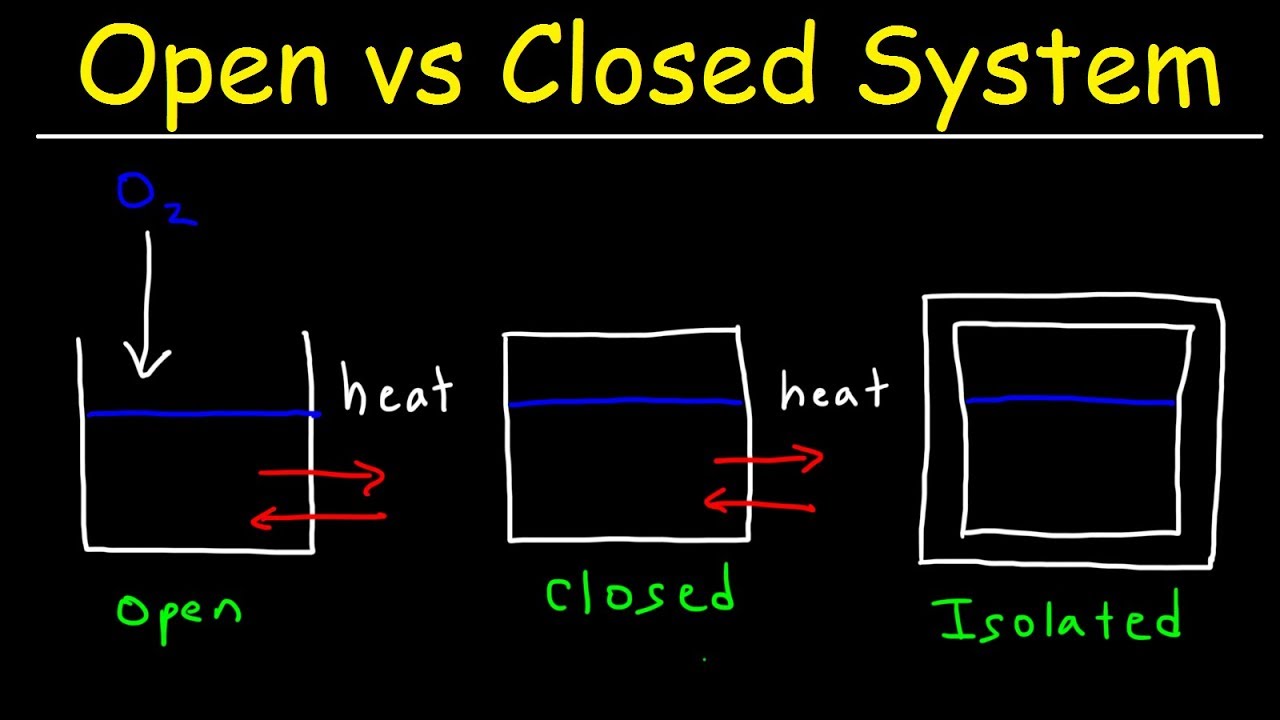
In a coffee cup calorimeter the reaction takes place in the water. Seems to me that most uses for constant pressure calorimetry coffee cup involve measuring enthalpy change in solutions whereas constant volume problems involve measuring enthalpy change from combustion reactions. Assume the final solution has a total volume of 100 mL. The coffee cup calorimeter is a a simplified DIY calorimeter but both serve the same purpose. Open System Closed System And Isolated System Thermodynamics Physics Thermodynamics Chemistry Education Physics.
 Source: pinterest.com
Source: pinterest.com
Explain why ΔH is obtained directly from a coffee cup calorimeter whereas ΔE is obtained directly from a bomb calorimeter. Top 4 posts. In a bomb calorimeter the reaction takes place in a sealed metal container which is the bomb vessel. We have the thermometer and the reaction mixture which are self explanatory along with the stirrer which stirs the reaction mixture. Understanding Coffee Cup And Bomb Calorimetry Chemistry Help Secondary Science Learning Science.
 Source: pinterest.com
Source: pinterest.com
What is the difference between bomb calorimeter and coffee cup calorimetry. With the coffee cup the specific heat of the solution is not the same as pure water so it makes determining the energy change more difficult than if you have the reaction closed off. A bomb calorimeter is used to measure heat flow for solids with low to high temperature reactions. The coffee cup calorimeter is a a simplified DIY calorimeter but both serve the same purpose. Best Growth Chamber Manufacturer And Supplier In India Laboratory Equipment Chamber Manufacturing.
 Source: in.pinterest.com
Source: in.pinterest.com
What is the difference between a coffee cup calorimeter and a bomb calorimeter in terms of the parameters. View Homework Help - bomb calorimetrydocx from CHEM 111 at University of South Carolina. In a bomb calorimeter the reaction takes place in a sealed metal container which is the bomb vessel. High temperature reactions because a styraphoam container is going to melt in high temperature so were not going to use that in high temperatures. Pin By Sara Yochannan On Saints Cooking With Kids Second Law Of Thermodynamics Food Science.
 Source: pinterest.com
Source: pinterest.com
Seems to me that most uses for constant pressure calorimetry coffee cup involve measuring enthalpy change in solutions whereas constant volume problems involve measuring enthalpy change from combustion reactions. Explain why ΔH is obtained directly from a coffee cup calorimeter whereas ΔE is obtained directly from a bomb calorimeter. What is the difference between a coffee cup calorimeter and a bomb calorimeter in terms of the parameters. View Homework Help - bomb calorimetrydocx from CHEM 111 at University of South Carolina. 10 Quick Calorimeter Tips Tricks Dds Calorimeters There Is No Doubt That Bomb Calorimeters Are The Most Valuable Instrument In A Laborato Dds Facts Reading.
 Source: pinterest.com
Source: pinterest.com
Trending Coffee Cup Calorimeter Vs Bomb Calorimeter A bomb calorimeter is 10 times larger but works the same way a bomb calorimeter measures heat for liquid products only a bomb calorimeter has a separate chamber to hold substances and can even measure heat gain or loss for reactions that do not occur in water. Bomb calorimeters are closed - they only allow heat energy to be exchanged volume is constant isochoric Coffee calorimeters are isolated only if they contain a lid that prevents gas escaping - they keep pressure constant and allow no heat exchange ideally adiabatic but gases matter can be exchanged depending on the set-up. Amethystcockroach626 Calorimetry is a method used to measure changes inenthalpy or heat that occur during chemical processes. CalorimetryChemistry AbdullahSanaullahTutorials Calorimetry CalorimeterCoffee CupCalorimeterBombCalorimeterClass11 NEET JEE MDCAT ECAT BestVi. Simple Homemade Calorimeter Science Activities For Toddlers Science Experiments Kids Science.
 Source: cz.pinterest.com
Source: cz.pinterest.com
Also why couldnt you just perform the former in a bomb calorimeter. Is there anything else to it than this. Trending Coffee Cup Calorimeter Vs Bomb Calorimeter A bomb calorimeter is 10 times larger but works the same way a bomb calorimeter measures heat for liquid products only a bomb calorimeter has a separate chamber to hold substances and can even measure heat gain or loss for reactions that do not occur in water. We have the thermometer and the reaction mixture which are self explanatory along with the stirrer which stirs the reaction mixture. Mhs 5200p Digital Dual Channel Dds Signal Generator Arbitrary Waveform Generator Function Signal Generator 6mhz Generator Office Phone Amplifier.
 Source: pinterest.com
Source: pinterest.com
A vigorous reaction occurs when a 0258 g piece of potassium solid is put in water inside a coffee cup calorimeter. We have a few parts to this coffee-cup calorimeter. In a coffee cup calorimeter the reaction takes place in the water. Bomb calorimeters are closed - they only allow heat energy to be exchanged volume is constant isochoric Coffee calorimeters are isolated only if they contain a lid that prevents gas escaping - they keep pressure constant and allow no heat exchange ideally adiabatic but gases matter can be exchanged depending on the set-up. Currents And Dc Circuits In 2021 Circuit Diagram Electric Lighter Simple Electric Circuit.
 Source: pinterest.com
Source: pinterest.com
Explain why ΔH is obtained directly from a coffee cup calorimeter whereas ΔE is obtained directly from a bomb calorimeter. View Homework Help - bomb calorimetrydocx from CHEM 111 at University of South Carolina. What is the difference between a coffee cup calorimeter and a bomb calorimeter in terms of the parameters. Seems to me that most uses for constant pressure calorimetry coffee cup involve measuring enthalpy change in solutions whereas constant volume problems involve measuring enthalpy change from combustion reactions. Burning Calories How Much Energy Is Stored In Different Types Of Food Science Project Science Fair Projects Fair Projects Food Science.
 Source: pinterest.com
Source: pinterest.com
Amethystcockroach626 Calorimetry is a method used to measure changes inenthalpy or heat that occur during chemical processes. Trending Coffee Cup Calorimeter Vs Bomb Calorimeter A bomb calorimeter is 10 times larger but works the same way a bomb calorimeter measures heat for liquid products only a bomb calorimeter has a separate chamber to hold substances and can even measure heat gain or loss for reactions that do not occur in water. With the bomb the reaction is closed off and you can measure just. Primarily a calorimeter is a tool utilized to estimate both specific heat capacity or the quantity of energy generated or consumed in a chemical reaction. Pin On Dds Blog Articles.
 Source: pinterest.com
Source: pinterest.com
View Homework Help - bomb calorimetrydocx from CHEM 111 at University of South Carolina. In a bomb calorimeter the reaction takes place in a sealed metal container which is the bomb vessel. Coffee cup calorimeter is much easier and probably used much often than a bomb calorimeter. In the textbookit is said that bomb calorimeter measures the constant volume heat while the cup calorimeter measure constant pressure heati was wondering why cup calorimeter is considered constant pressureeven though it also does not allow a gas to expand it has a fixed volumeso if not then that means cup calorimeter allows some air to. Understanding Coffee Cup And Bomb Calorimetry Lab Equipment Laboratory Equipment Heat Change.
 Source: pinterest.com
Source: pinterest.com
Postby Jessica Benitez 1K Mon Jan 15 2018 712 am The bomb calorimeter has constant volume while the coffee cup calorimeter has constant pressure. What is the difference between a coffee cup calorimeter and a bomb calorimeter in terms of the parameters. A vigorous reaction occurs when a 0258 g piece of potassium solid is put in water inside a coffee cup calorimeter. This chemistry video tutorial highlights the difference between the bomb calorimeter and the coffee cup calorimeter. The Difference Between Calories In Foodstuff Calories In Combustible Material Dds Calorimeters Foodstuff Calorie Material.







